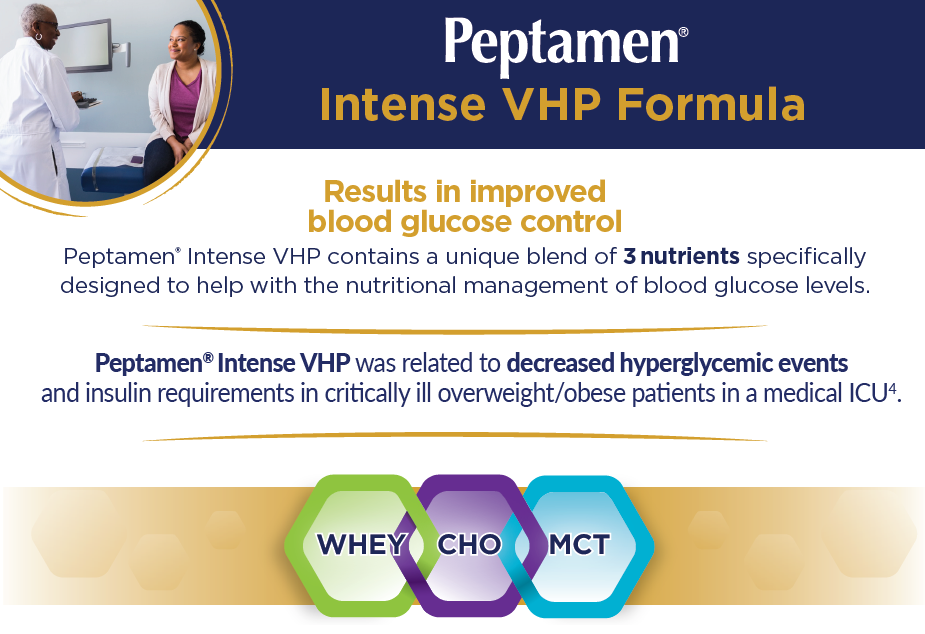
Use of Peptamen® formulas in critically ill patients was associated with lower GI intolerance and greater glycemic control, relative to use of other formulas.
In a retrospective study of 60 pediatric and adult patients in a post-acute care setting diagnosed with illnesses such as malignancy, hepato-biliary/pancreatic disorders and other GI conditions, use of Peptamen® formulas was associated with a 50.1% reduction in enteral feeding intolerance and a 45.5% decrease in cost of care.

Study Summary
Characteristics and Feeding Intolerance in Critically Ill Adult Patients Receiving Peptide-Based Enteral Nutrition: A Retrospective Cross-Sectional Study
Douglas Nguyen, Laura Schott, Cynthia Lowen, Amarsinh Desai, Dorothy Baumer, Mary Miranowski, Zhun Cao, Krysmaru Araujo Torres
Clinical Nutrition ESPEN. 2024;59:270-278.
Background
Gastrointestinal intolerance and hyperglycemia, often associated with enteral nutritional support, may lead to inadequate delivery of nutrients and related poor clinical outcomes.
Objective
The objective of this real world study was to examine characteristics, disease severity and enteral nutrition (EN) formulas as they related to enteral feeding intolerance (EFI) and healthcare resource utilization (HCRU) in adult critically ill patients.
Methods
This retrospective, cross-sectional study used real world data from PINC AITM Healthcare Database representing 25% of US hospitals and captured information from the years 2015-2019. Adult hospitalized patients who were critically ill and received ≥3 days of EN were included. Comparator groups were comprised of those patients who received 100% whey-peptide EN, other peptide-based EN, and intact protein standard or diabetic EN formulas. Primary outcomes included assessment of EFI, both GI intolerance and hyperglycemia, as related to type of formula received. GI intolerance was defined as presence of abdominal distention, abdominal pain, constipation, diarrhea, nausea or vomiting.
Results
There were 19,679 inpatients from 67 US hospitals included in this study, receiving a median of 6 days of EN. Breakdown of formulas received were as follows:
- 3,242 inpatients received 100% whey-peptide EN
- 3,121 inpatients received other peptide-based EN
- 13,316 inpatients received standard or diabetic intact-protein EN
The 100% whey-peptide EN group had higher frequencies of co-morbidities and severity of illness, as compared to the other EN groups.
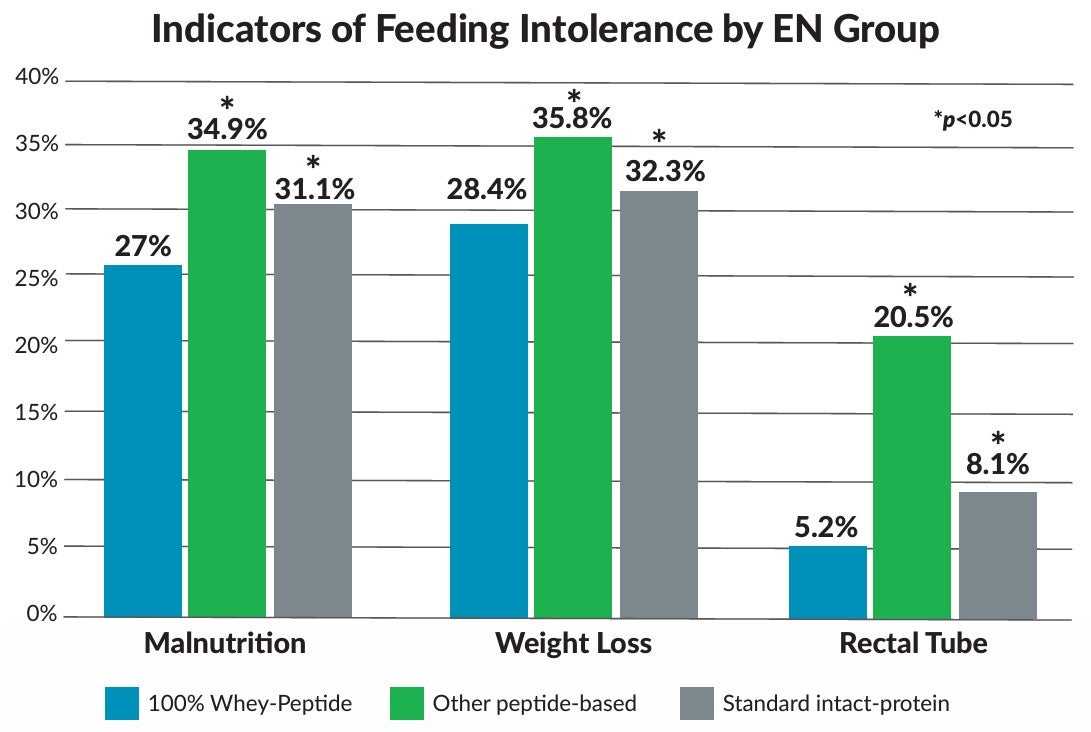
After controlling for severity characteristics, visit and demographics, odds of GI intolerance were 18% higher for the other peptide-based group and 15% higher for the standard intact-protein group, as compared to the 100% whey-peptide EN group. Odds of hyperglycemia were 81% higher for the other peptide-based group versus the very high protein/low carbohydrate 100% whey-peptide group.
Conclusion
■ This largest study to date, describing EN formula selection in critically ill adults in the US, showed the advantages to using 100% whey peptide EN formula, which included lower frequencies of GI intolerance, malnutrition, weight loss and need for rectal tube.
Use of 100% whey peptide may benefit critically ill patients by reducing EN feeding intolerance and enhancing nutrient assimilation. In addition, odds of hyperglycemia may be lowered with use of a very high protein/low carbohydrate 100% whey peptide EN formula.
To request samples and find out more information contact your Nestlé Health Science representative, call 1-800-422-ASK2 (2752), or visit www.NestleMedicalHub.com/brands/peptamen.
Helpful Links:
Download the PDF version of this Peptamen Power Pack
Link to journal article: https://sciencedirect.com/science/article/pii/S2405457723022337
Nguyen N, et al. Characteristics and feeding intolerance in critically ill adult patients receiving peptide-based enteral nutrition: A retrospective cross-sectional study. Clinical Nutrition ESPEN. 2024;59:270-278.
This study was funded by Nestlé Health Science.
USE UNDER MEDICAL SUPERVISION.
This information is for educational purposes only and is not intended as a substitute for medical advice.
All trademarks are owned by Société des Produits Nestlé S.A., Vevey, Switzerland.
©2024 Nestlé. All rights reserved.