Compleat® Peptide 1.5 formula with fruit and vegetable ingredients was found to be associated with significant reductions in GI intolerance symptoms.
In a retrospective observational study of 82 post-acute care adult patients (≥14 years) with medical comorbidities, use of COMPLEAT® Peptide 1.5 formula made with fruit and vegetable ingredients was found to be well tolerated and associated with significant reductions in GI intolerance symptoms.

Poster Summary
Enhanced Gastrointestinal Tolerance in Post-Acute Care Adults Receiving a Plant-Based Peptide Enteral Formula: A Retrospective Analysis
1 Medical Affairs, Nestlé Health Science, Bridgewater, New Jersey
2 Market Access, Nestlé Health Science, Bridgewater, New Jersey
3 Clarivate Data Analytics & Insights, Bangalore, India
Introduction
Enteral nutrition (EN) is the standard of care for patients with a functional gastrointestinal (GI) tract who are unable to meet nutrition goals by mouth. For patients with intolerance to standard polymeric enteral formulas, peptide-based EN containing hydrolyzed protein, specifically designed to enhance digestion and absorption, may be indicated.1-3 There is a growing body of evidence supporting improved GI tolerance with either peptide-based EN or formulas containing real fruit and vegetable ingredients.3,4 However, there is a paucity of evidence on plant- and peptide-based EN also containing real fruit and vegetable ingredients.
Objectives
To examine the clinical outcomes related to GI intolerance in adults in the post-acute care setting receiving a plant-based peptide EN formula containing real fruit and vegetable ingredients.
Methods
This retrospective study of de-identified data was conducted using nationally representative US claims data from the Clarivate Real World Evidence Data Repository, which links medical and prescription claims and electronic health records to provide longitudinal patient-level data representative of more than 300 million patients in the United States (US). This repository covers 98% of US health plans. Post-acute care adults (≥14 years) prescribed a plant-based peptide (hydrolyzed pea protein) EN formula containing real fruit and vegetable ingredients (Compleat® Peptide 1.5, Nestlé HealthCare Nutrition, US; [PPF]) between January 2020 and December 2022 were included. The index date was defined as the date of hospital discharge, the pre-index period was defined as 6 months prior to the index date, and the post-index periods as 1, 3 and 6 months (24, 84 and 168 days, respectively) after the index date. GI intolerance symptoms at pre-and post-index periods were compared with results presented as mean (SD) or n (%) and comparisons conducted using a Chi-squared test.
Patient Characteristics
The study included 82 adult patients (56% female; mean age 49 (SD±20.5 years) representative of all US regions. Among the 75 patients (91%) with pre-index comorbidities, the most common conditions were cancer (39%), chronic pulmonary disease (29%), paraplegia and hemiplegia (24%) and mild liver disease (24%). The mean (SD) Charlson Comorbidity Index (CCI)5 weighted score was 7.2 (4.6), and 56% of patients with comorbidities had a CCI ≥3. The most common medications prescribed pre-index were central nervous system agents (59%), GI drugs (39%) and anti-infective agents (36%).
Results
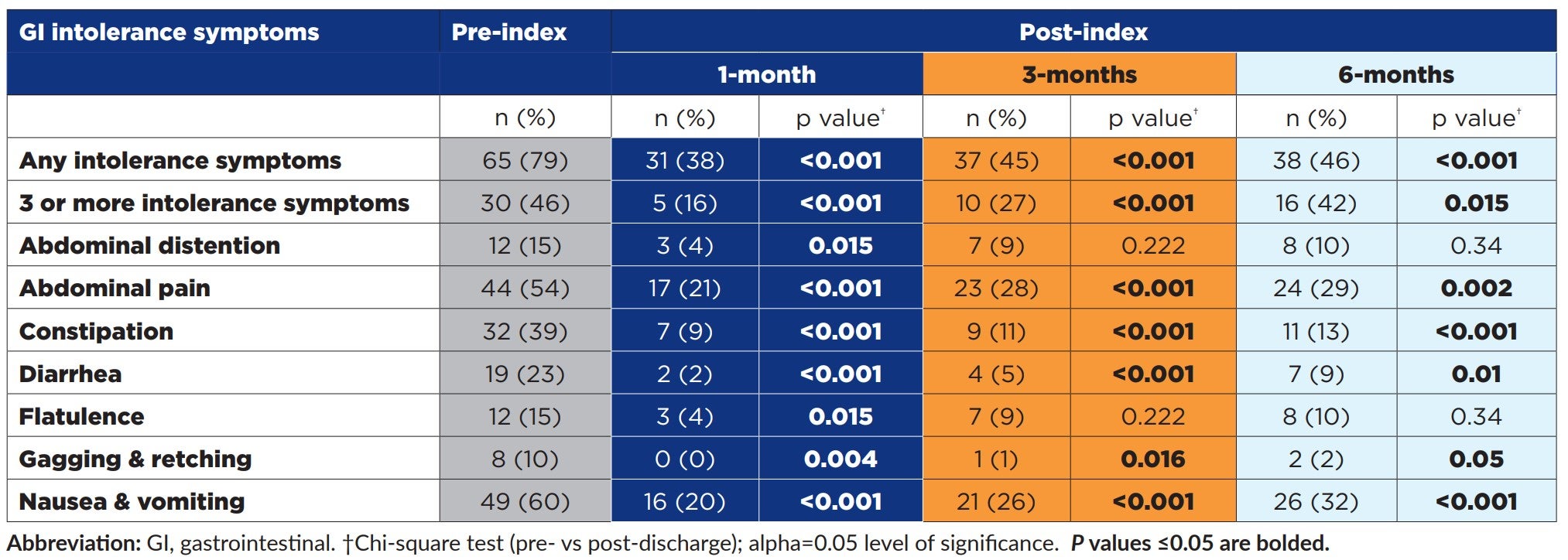
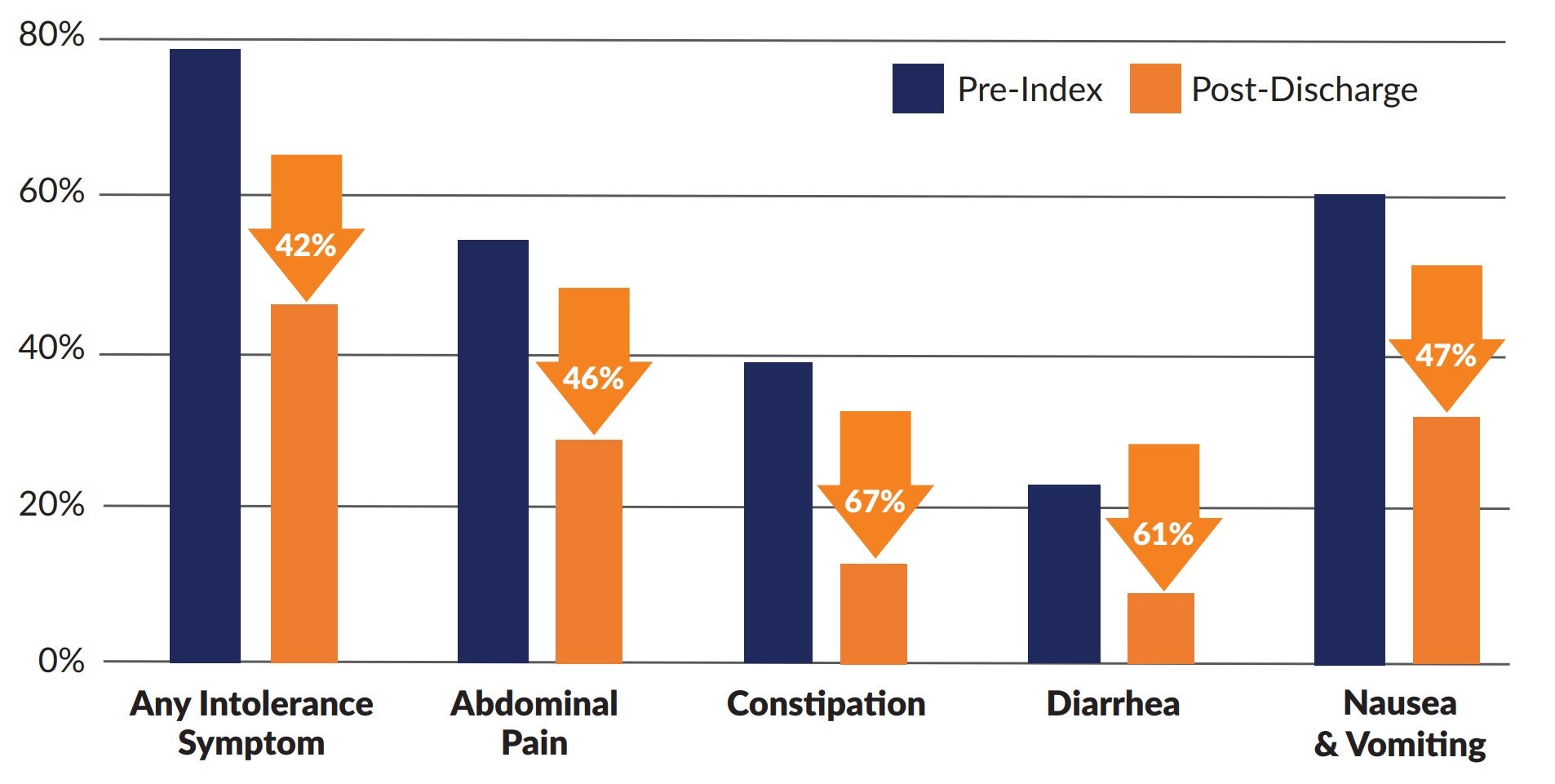
Compared to pre-index, significantly fewer patients experienced any GI intolerance symptoms at all post-index time points (p<0.001), see Table. Likewise, significantly fewer patients experienced 3 or more intolerance symptoms at all post-index time points compared to pre-index (p<0.001). Significant reductions were observed in individual GI symptoms such as abdominal pain, constipation, diarrhea, gagging & retching, and nausea & vomiting at all post-index time points evaluated (all p≤0.05).
Intolerance Among Post-Acute Adults Receiving a Plant-Based Peptide EN Formula Containing Real Fruit and Vegetable Ingredients
Conclusion
Use of a plant-based peptide EN formula containing real fruit and vegetable ingredients was associated with significant reductions in any GI intolerance symptoms up to 6 months post hospital discharge. These improvements support PPF as a well-tolerated formula in adults requiring enteral nutrition support in a post-acute care setting.
References:
1. Mohamed Elfadil O, et al. Nutr Clin Pract. 2023 Apr;38(2):318-328.
2. LaVallee C, et al. JPEN J Parenter Enteral Nutr. 2021;45(8):1729-1735.
3. Mohamed Elfadil O, et al. JPEN J Parenter Enteral Nutr. 2022;46(3):626-634.
4. Adams RL. Journal of Food and Nutritional Sciences. 2021;31(1):18-33.
5. Charlson ME, et al. J Chronic Dis. 1987;40(5):373-383
To request samples and find out more information contact your Nestlé Health Science representative, call 1-800-422-ASK2 (2752), or visit www.NestleMedicalHub.com/brands/compleat.
Helpful Links:
Summary prepared by Nestlé Health Science. Download PDF of Summary
The poster presented at ASPEN 2024 may be accessed online: Poster 34
Access the Compleat® Peptide 1.5 Power Pack
Unless otherwise indicated, all trademarks are owned by Société des Produits Nestlé S.A., Vevey, Switzerland. ©2024 Nestlé. All rights reserved. Bridgewater, NJ 08807 U.S.A