Compleat® Pediatric Peptide 1.5 and Compleat® Peptide 1.5 made with real fruit and vegetable ingredients are associated with reductions in healthcare resource utilization and cost savings.

In a retrospective, observational study of 91 children and 82 adults in the post-acute care setting, Compleat® Pediatric Peptide 1.5 and Compleat® Peptide 1.5 made with real fruit and vegetable ingredients were associated with significant reductions in healthcare resource utilization and cost savings up to 6 months post-hospital discharge compared to the 6 months prior to hospital discharge.
Poster Summary
Healthcare Resource Utilization and Costs Associated with a Peptide-Based Enteral Formula with Fruit and Vegetable Ingredients: Retrospective Analysis of Children and Adults in Post-Acute Care
1Market Access, Nestlé Health Science; 2Medical Affairs, Nestlé Health Science; 3Clarivate Data Analytics and Insights, Bangalore, Karnataka, India; 4Clarivate Analytics Ltd, London, UK
Value in Health, Volume 27, Issue 6, S1(June 2024).
Introduction
Enteral nutrition (EN) is standard of care for patients unable to meet nutrition needs orally, with peptide-based EN indicated when intolerance to standard polymeric EN is present.1-3 Evidence supports improved gastrointestinal tolerance with plant- and peptide-based EN made with real fruit and vegetable ingredients4,5; however, data are scarce regarding health economic impacts associated with these formulas.
Objectives
To examine healthcare resource utilization (HCRU) and associated costs in children and adults receiving plant-based peptide formulas containing fruit and vegetable ingredients in post-acute care.
Methods
This retrospective, observational study of de-identified data was conducted using nationally representative United States (US) claims data from the Decisions Resources Group Real World Evidence Data repository, which links medical and prescription claims and electronic health records to provide longitudinal patient-level data representative of more than 300 million patients in the US. Post-acute care children (1-13 years) and adults (≥14 years) prescribed a plant-based peptide (hydrolyzed pea protein) EN formula containing real fruit and vegetable ingredients (Compleat® Pediatric Peptide 1.5, Compleat® Peptide 1.5, Nestlé US) as sole source nutrition for ≥5 days between January 2020 and December 2022 were included. The index date was defined as the date of hospital discharge, the pre-index period was defined as 6 months prior to the index date, and the post-index periods as 1, 3, and 6 months (24, 84 and 168 days, respectively) after the index date. Healthcare resource utilization was compared at preand post-index periods for inpatient, outpatient, urgent care (UC), emergency department (ED), telemedicine, other places of service and total visits. Multivariate costs, after adjusting for age, sex, and comorbidity index scores, were compared in pre-and post-index periods.
Patient Characteristics
For children (n=91), 49% were female with mean (SD) age of 5.5 (3) and an overall mean (SD) Pediatric Comorbidity Index (PCI)6 score of 7.4 (3.5). Most PCI scores were ≥4, indicating a higher severity of comorbidity, and the most common comorbidities were congenital malformations (73%), developmental delays (60%), and gastrointestinal conditions (57%). For adults (n=82), 56% were female with a mean (SD) age of 49 (20.5) and an overall mean (SD) Charlson Comorbidity Index (CCI)7 weighted score of 7.2 (4.6). The majority of CCI weighted scores were ≥3 indicating moderate severity of comorbidity. The most common comorbidities were cancer (39%), chronic pulmonary disease (29%) and paraplegia and hemiplegia (24%). In both the pediatric and adult cohorts, all US regions were represented.
Results
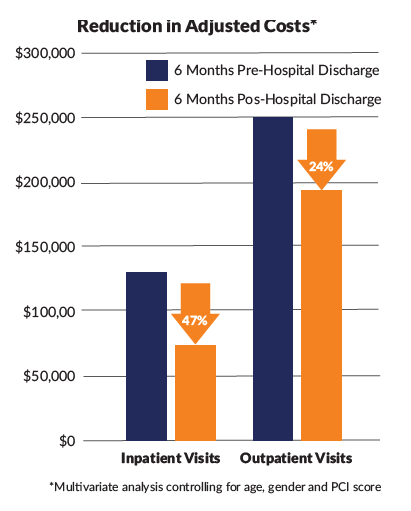
In the pediatric cohort, mean outpatient visits were reduced at all postindex periods and mean total visits up to 3 months post-index (p≤0.05). Differences in mean visits for inpatient, ED and UC were not statistically significant. The proportion of children requiring inpatient visits was significantly reduced at all post-index periods (p≤0.05). HCRU reductions resulted in significant decreases in mean adjusted costs associated with inpatient and outpatient visits at all post-index periods; when comparing pre-index to the six month post index period, mean adjusted costs for inpatient visits were $127,371 vs $67,507 and for outpatient visits $255,792 vs $193,784, p≤0.05. Total HCRU mean adjusted costs were reduced up to 3 months post-index (pre-index $473,857 vs 3-month post index $273,134), p≤0.05.
In the adult cohort, mean outpatient visits were significantly reduced at all post-index periods, and mean ED, other place of service, and total visits up to 3 months post-index (p≤0.05). Mean inpatient visits were reduced up to 1 month, while UC and telemedicine were not significantly different. The proportion of adults requiring inpatient visits was significantly reduced at all post-index periods, and other visits up to 3 months (p≤0.05). For ED visits reductions were observed up to 1 month, with no differences for remaining visit types. HCRU reductions resulted in significant decrease of total mean adjusted costs and mean adjusted costs associated with outpatient, inpatient, ED, and UC visits at all post index periods (p≤0.05). When comparing pre-index to the six month post index period, mean adjusted costs for inpatient visits were $135,783 vs $82,827, outpatient visits $398,180 vs $235,996, and total HCRU $676,456 vs $427,576, all p≤0.05.
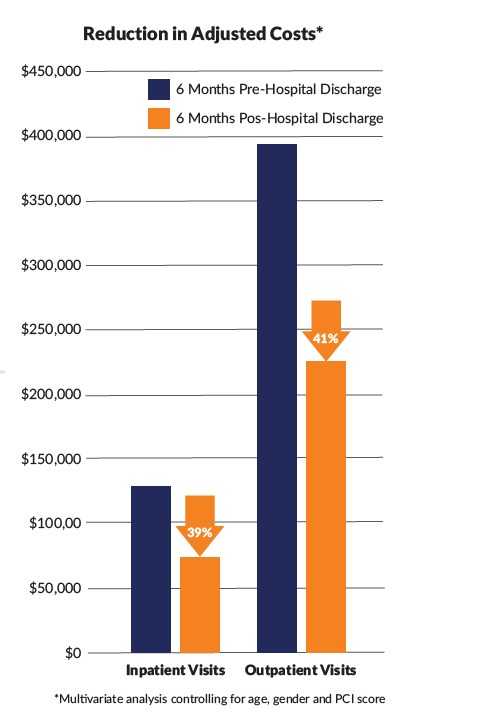
Conclusion
Use of a plant-based peptide EN formula containing fruit and vegetable ingredients was associated with significant reductions in various HCRU measures up to 6 months post hospital discharge, compared to the 6 month period prior to discharge, in children and adults with most consistent reductions observed 1-month post index. These improvements in HCRU support use of these formulas as a cost savings option in children and adults requiring peptide-based EN in a post-acute care setting.
References:
1. Nutr Clin Pract. 2023 Apr;38(2):318-328.
2. JPEN J Parenter Enteral Nutr. 2021;45(8):1729-1735.
3. JPEN J Parenter Enteral Nutr. 2022;46(3):626-634.
4. Pediatr Gastroenterol Nutr. 2023;77(1):S551-552
5. J Parenter Enteral Nutr. 2024; 48:S114-116.
6. Am J Epidemiol. 2021 May 4; 190(5):918-927.
7. J Chronic Dis. 1987;40(5):373-38
To request samples and find out more information contact your Nestlé Health Science representative, call 1-800-422-ASK2 (2752), or visit www.NestleMedicalHub.com/brands/compleat.
Helpful Links:
Summary prepared by Nestlé Health Science. Download PDF of Summary
The poster presented at ISPOR 2024 may be accessed online: ISPOR Poster
Access the Compleat® Peptide 1.5 ISPOR Power Pack
Unless otherwise indicated, all trademarks are owned by Société des Produits Nestlé S.A., Vevey, Switzerland. ©2024 Nestlé. All rights reserved. Bridgewater, NJ 08807 U.S.A